
Chemistry, 27.03.2020 01:50 marsewilliams
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected.
UO2(s) + 4 HF(g) ↔ UF4(g) + 2 H2O(g)
1. Water vapor is removed
2. UF4(g) is added
3. The reaction is done in a glass reaction vessel. HF(g) attacks and reacts with the glass
4. If the reaction is endothermic, and you want to make the equilibrium shift to the right to maximize the products, would you heat or cool the reaction vessel?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of t...
Questions













Geography, 23.07.2019 04:20




Mathematics, 23.07.2019 04:20

Mathematics, 23.07.2019 04:20

Mathematics, 23.07.2019 04:20

Computers and Technology, 23.07.2019 04:20

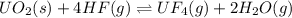
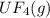 is added
is added

