
Chemistry, 27.03.2020 01:29 mcckenziee
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2 ( s ) − ⇀ ↽ − M ( s ) + O 2 ( g ) Δ G ∘ = 291.0 kJ mol When this reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. What is the chemical equation of this coupled process? Show that the reaction is in equilibrium. Include physical states and represent graphite as C ( s ) .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

You know the right answer?
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2...
Questions


Mathematics, 01.06.2021 21:50


History, 01.06.2021 21:50

Mathematics, 01.06.2021 21:50

World Languages, 01.06.2021 21:50




Social Studies, 01.06.2021 21:50


Social Studies, 01.06.2021 21:50




Mathematics, 01.06.2021 21:50

Physics, 01.06.2021 21:50

Mathematics, 01.06.2021 21:50

Mathematics, 01.06.2021 21:50

English, 01.06.2021 21:50

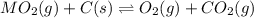
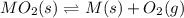 .....[1]
.....[1]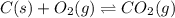 ..[2]
..[2]

