
Chemistry, 27.03.2020 01:00 braydenmcd02
Calculate the pKa of acetic acid and the pKb of methylamine (CH3NH2). Acetic acid’s Ka is 1.8 x 10-5. Methylamine’s Ka is 4.2 x 10-4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Calculate the pKa of acetic acid and the pKb of methylamine (CH3NH2). Acetic acid’s Ka is 1.8 x 10-5...
Questions

History, 25.03.2021 05:50


Mathematics, 25.03.2021 05:50

Chemistry, 25.03.2021 05:50

Mathematics, 25.03.2021 05:50


Chemistry, 25.03.2021 05:50

Advanced Placement (AP), 25.03.2021 05:50

Computers and Technology, 25.03.2021 05:50


Mathematics, 25.03.2021 05:50

English, 25.03.2021 05:50



Chemistry, 25.03.2021 05:50



Mathematics, 25.03.2021 05:50

Mathematics, 25.03.2021 05:50

History, 25.03.2021 05:50

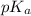 of acetic acid is 4.7.
of acetic acid is 4.7.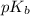 of methylamine is 3.4.
of methylamine is 3.4.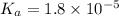
![pK_a=-\log[K_a]](/tpl/images/0566/4951/78bbf.png)
![pK_a=-\log[1.8\times 10^{-5}]=4.7](/tpl/images/0566/4951/353fe.png)
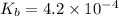
![pK_b=-\log[K_b]](/tpl/images/0566/4951/b72fe.png)
![pK_b=-\log[4.2\times 10^{-4}]=3.4](/tpl/images/0566/4951/6fbec.png)


