Chemistry, 26.03.2020 23:42 nadarius2017
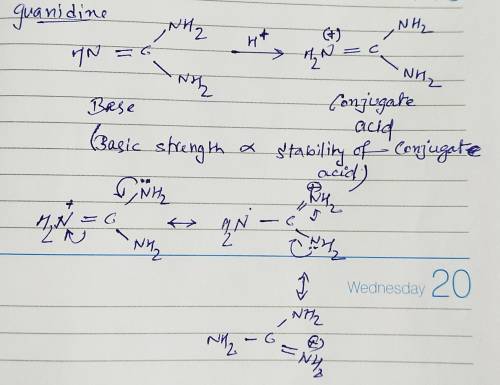
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by drawing the resonance structures of the protonated guanidine. The protonated guanidine (A) has been drawn for you. Draw major resonance structures, one each in boxes B and C, and one minor resonance structure in Box D. Be sure to include the formal charge, lone pairs, and hydrogens on nitrogen for structures B, C, and D.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by draw...
Questions



English, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00

Biology, 02.08.2019 15:00

English, 02.08.2019 15:00

Geography, 02.08.2019 15:00


Geography, 02.08.2019 15:00


Health, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00

Social Studies, 02.08.2019 15:00

Mathematics, 02.08.2019 15:00