
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution at 22°C. Which statement explains why the contents of the flask are at equilibrium?The rate of dissolving is equal to the rate of crystallization. The rate of dissolving is greater than the rate of crystallization. The concentration of the solid is equal to the concentration of the solution. The concentration of the solid is greater than the concentration of the solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution...
Questions

Spanish, 19.08.2020 14:01



Mathematics, 19.08.2020 14:01

Biology, 19.08.2020 14:01

History, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01

History, 19.08.2020 14:01

Geography, 19.08.2020 14:01


Mathematics, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01


Mathematics, 19.08.2020 14:01

History, 19.08.2020 14:01

Mathematics, 19.08.2020 14:01


Spanish, 19.08.2020 14:01

History, 19.08.2020 14:01

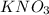 from aqueous
from aqueous 

