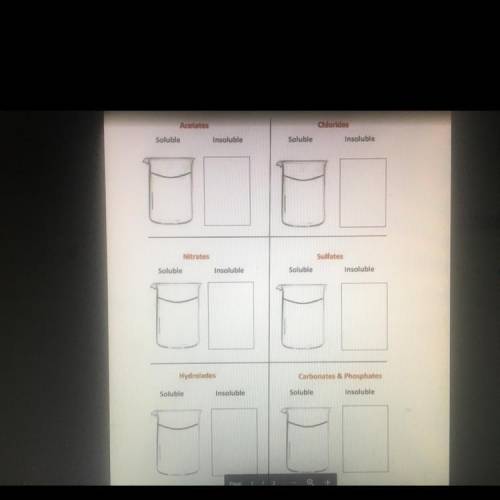
After identifying the anions (negative ions), in each of the following
compounds, write the fo...

Chemistry, 26.03.2020 23:27 johnkhan6748
After identifying the anions (negative ions), in each of the following
compounds, write the formula for the compound in either the soluble
beaker or insoluble box in the correct anion section, on the other page.
AgCl
NaCzH302
Lici
H2SO4
Pb(NO3)2
CaCO3
NaOH
Ca(OH)2
КОН
Cu(OH)2
CaSO4
HCI
(NH4)3PO4
Ca(NO3)2
MgCO3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Questions

Mathematics, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31


Mathematics, 21.11.2019 07:31


English, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31




Mathematics, 21.11.2019 07:31

History, 21.11.2019 07:31

History, 21.11.2019 07:31


History, 21.11.2019 07:31

English, 21.11.2019 07:31


Mathematics, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31



