
Chemistry, 26.03.2020 22:48 giraffesaur44
Determine the expression for the rate of the reaction with respect to each of the reactants and products. Determine the expression for the rate of the reaction with respect to each of the reactants and products. Rate=−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]ΔtRate =−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]Δt Rate=−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]ΔtRate =−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]Δt Rate=−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]ΔtRate =−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]Δt Rate=12Δ[A]Δt=12Δ[B]Δt=13Δ[C]Δt

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Determine the expression for the rate of the reaction with respect to each of the reactants and prod...
Questions



English, 01.09.2019 20:30

Chemistry, 01.09.2019 20:30



History, 01.09.2019 20:30




Social Studies, 01.09.2019 20:30



History, 01.09.2019 20:30



Mathematics, 01.09.2019 20:30


Social Studies, 01.09.2019 20:30

Advanced Placement (AP), 01.09.2019 20:30

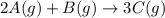
![Rate=-\frac{1}{3}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{2}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/ff0f8.png)
![Rate=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/11b91.png)
![Rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/f7a0b.png)
![Rate=\frac{1}{2}\frac{\Delta [A]}{\Delta t}=\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/3152a.png)
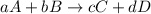
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0566/1120/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0566/1120/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0566/1120/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/d4b94.png)
![\text{Rate of disappearance of }A=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}](/tpl/images/0566/1120/4cd85.png)
![\text{Rate of disappearance of }B=-\frac{\Delta [B]}{\Delta t}](/tpl/images/0566/1120/43240.png)
![\text{Rate of formation of }C=+\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/d1447.png)


