
Chemistry, 26.03.2020 22:42 elijahsantiago21
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 mL of a CH3COOH (acetic acid)/ CH3COONa * 3H2O buffer. The target pH of the buffer is 5.25. The given concentration of [CH3COOH] is equal to 0.10 M. Ka = 1.80 x 10-5 for acetic acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 m...
Questions

Biology, 07.11.2020 01:40



Mathematics, 07.11.2020 01:40





Arts, 07.11.2020 01:40

Arts, 07.11.2020 01:40



Mathematics, 07.11.2020 01:40

Mathematics, 07.11.2020 01:40

Mathematics, 07.11.2020 01:40


Chemistry, 07.11.2020 01:40

Physics, 07.11.2020 01:40

Mathematics, 07.11.2020 01:40

Mathematics, 07.11.2020 01:40

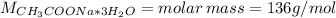
![pH = pKa + log(\frac{[CH_{3}COONa*3H_{2}O]}{[CH_{3}COOH]})](/tpl/images/0566/0820/ec252.png) (1)
(1)![log [CH_{3}COONa*3H_{2}O] = pH - pKa + log [CH_{3}COOH]](/tpl/images/0566/0820/d2774.png)
![log [CH_{3}COONa*3H_{2}O] = 5.25 - (-log(1.80 \cdot 10^{-5})) + log (0.10) = -0.495](/tpl/images/0566/0820/9589c.png)
![[CH_{3}COONa*3H_{2}O] = 10^{-0.495} = 0.32 M](/tpl/images/0566/0820/c6ecf.png)
![m = moles*M = [CH_{3}COONa*3H_{2}O]*V*M = 0.32 mol/L*0.250 L*136 g/mol = 10.88 g](/tpl/images/0566/0820/427f5.png)


