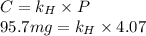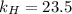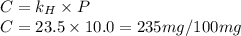Chemistry, 26.03.2020 22:40 xxgissellexx
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g water . Calculate the solubility of N2 gas in water, at the same temperature, if the partial pressure of N2 gas over the solution is increased from 4.07 atm to 10.0 atm .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g w...
Questions



English, 26.02.2022 16:10


History, 26.02.2022 16:10


Social Studies, 26.02.2022 16:10

Mathematics, 26.02.2022 16:10


Computers and Technology, 26.02.2022 16:10

Social Studies, 26.02.2022 16:10

Chemistry, 26.02.2022 16:10





Mathematics, 26.02.2022 16:10



 gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.
gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.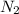 in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.
in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.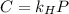 .
.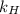 = Henry’s law constant = ?
= Henry’s law constant = ?