
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 10. g of octane is mixed with 61.9 g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions


Mathematics, 19.09.2019 16:30





Geography, 19.09.2019 16:30




Computers and Technology, 19.09.2019 16:30

Mathematics, 19.09.2019 16:30






English, 19.09.2019 16:30


Business, 19.09.2019 16:30

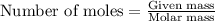
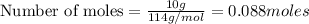
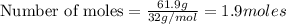
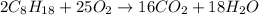
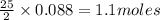 of ethane
of ethane

