
Chemistry, 26.03.2020 22:01 kharmaculpepper
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the rate of disappearance of Cl2 is 4.44 × 10-2 M/s, what is the rate of disappearance of NO? 2 NO(g) + Cl2(g) → 2 NOCl(g) 2.22 × 10-2 M/s 1.11 × 10-1 M/s 4.44 × 10-2 M/s 8.88 × 10-2 M/s 5.25 × 10-2 M/s

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [Cl2]. If the...
Questions


Chemistry, 31.08.2019 07:00

Chemistry, 31.08.2019 07:00

Mathematics, 31.08.2019 07:00

Biology, 31.08.2019 07:00

History, 31.08.2019 07:00

History, 31.08.2019 07:00

Biology, 31.08.2019 07:00

Physics, 31.08.2019 07:00

Mathematics, 31.08.2019 07:00

English, 31.08.2019 07:00

History, 31.08.2019 07:00

History, 31.08.2019 07:00


Mathematics, 31.08.2019 07:00

Mathematics, 31.08.2019 07:00

English, 31.08.2019 07:00

Biology, 31.08.2019 07:00


Computers and Technology, 31.08.2019 07:00

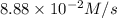
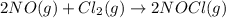
![-\frac{1d[NO]}{2dt}=-\frac{d[Cl_2]}{dt}=+\frac{1d[NOCl]}{2dt}](/tpl/images/0565/9272/04d4d.png)
![\frac{-d[Cl_2]}{dt}]=4.44\times 10^{-2}M/s](/tpl/images/0565/9272/271c7.png)
![-\frac{1d[NO]}{dt}=2\times {\frac{-d[Cl_2]}{dt}=2\times 4.44\times 10^{-2}M/s=8.88\times 10^{-2}M/s](/tpl/images/0565/9272/dea34.png)


