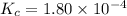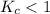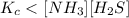Chemistry, 26.03.2020 21:33 demienarravo
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) This reaction is Reactant favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2...
Questions






History, 07.05.2020 02:08




Mathematics, 07.05.2020 02:09

Mathematics, 07.05.2020 02:09




Mathematics, 07.05.2020 02:09


History, 07.05.2020 02:09

Biology, 07.05.2020 02:09


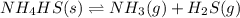
![K_c=[NH_3][H_2S]](/tpl/images/0565/8253/ff5ed.png)
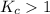 ; the reaction is product favored.
; the reaction is product favored.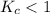 ; the reaction is reactant favored.
; the reaction is reactant favored.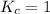 ; the reaction is in equilibrium.
; the reaction is in equilibrium.