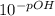Chemistry, 02.11.2019 10:31 samantha9014
Calculate the hydroxide ion consentration of a solution with ph = 3.25. show all calculations leading to your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Calculate the hydroxide ion consentration of a solution with ph = 3.25. show all calculations leadin...
Questions




Mathematics, 12.11.2020 07:30

Chemistry, 12.11.2020 07:30

English, 12.11.2020 07:30


Mathematics, 12.11.2020 07:30


Mathematics, 12.11.2020 07:30

Chemistry, 12.11.2020 07:30

Biology, 12.11.2020 07:30

English, 12.11.2020 07:30


English, 12.11.2020 07:30



Mathematics, 12.11.2020 07:30