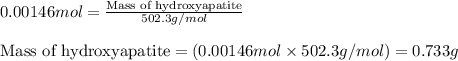Chemistry, 26.03.2020 20:31 sparky1234
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to toothpaste it reacts with the enamel to produce a more decay-resistant material fluoroapatite ( Ca5(PO4)3F. The by-products of this reaction are tin(II)oxide and water. What mass of hydroxyapatite can be converted to fluoroapatite by reaction with 0.115 grams of tin(II)fluoride

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
1. Tooth enamel consists mainly of hydroxyapatite (Ca5(PO4)3(OH). When tin(II)fluoride is added to t...
Questions

Geography, 20.09.2019 20:00

Biology, 20.09.2019 20:00





Biology, 20.09.2019 20:00

Social Studies, 20.09.2019 20:00

Mathematics, 20.09.2019 20:00

Geography, 20.09.2019 20:00



Mathematics, 20.09.2019 20:00

Mathematics, 20.09.2019 20:00




History, 20.09.2019 20:00


Mathematics, 20.09.2019 20:00

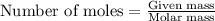 .....(1)
.....(1)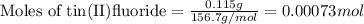
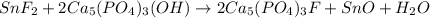
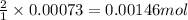 of hydroxyapatite
of hydroxyapatite