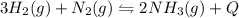In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is ex...

Chemistry, 10.10.2019 16:30 jacobbecker99
In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is exothermic. which set of conditions would give the best yield of
ammonia at equilibrium?
a) 800 atmospheres and 2000 oc
b) 1 atmosphere and 500 o
c
c) 1 atmosphere and 2000 o
c
d) 800 atmospheres and 500 o
c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Questions



History, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30






Arts, 06.11.2020 19:30

Health, 06.11.2020 19:30


Mathematics, 06.11.2020 19:30

Computers and Technology, 06.11.2020 19:30



Mathematics, 06.11.2020 19:30


Mathematics, 06.11.2020 19:30