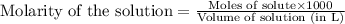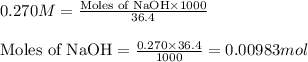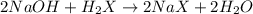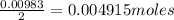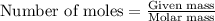A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is the molar mass of the acid if 36.4 mL of the NaOH solution is required to neutralize the sample? Assume the volume of NaOH corresponds to the second equivalence point. A flask with a solution sits on the base of a ring stand. A buret filled with liquid is suspended above the flask by the ring stand. molar mass: g/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is th...
Questions

Biology, 02.11.2020 23:50

Business, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

Health, 02.11.2020 23:50

History, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50


Geography, 02.11.2020 23:50

Geography, 02.11.2020 23:50



Mathematics, 02.11.2020 23:50


Mathematics, 02.11.2020 23:50


Health, 02.11.2020 23:50

Biology, 02.11.2020 23:50