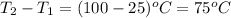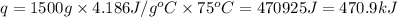How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL for the water.)
My brain wants to just start with 1.5L and use density as a conversion factor but I seriously think I'm missing something. I don't really understand the heat equation, but I'm thinking I might need to use q = mCAT?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL...
Questions


Mathematics, 04.02.2020 01:56

Mathematics, 04.02.2020 01:56

History, 04.02.2020 01:56

Mathematics, 04.02.2020 01:56



Mathematics, 04.02.2020 01:56

Social Studies, 04.02.2020 01:56


History, 04.02.2020 01:56

Spanish, 04.02.2020 01:56


Computers and Technology, 04.02.2020 01:56






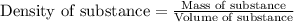
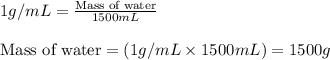
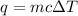
 = change in temperature =
= change in temperature =