
Chemistry, 26.03.2020 20:02 kaleahlove13
Name: Date: Period: 3/23 - 3/27 Assignment 1: Gas Law Practice Problems 1. A gas occupies 12.3 liters at a pressure of 40.0 mm Hg. What is the volume when the pressure is increased to 60.0 mm Hg? Given: Equation: 2. A gas occupies 900.0 mL at a temperature of 27.0 °C. What is the volume at 132.0 °C? Given: Equation: 3. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its original temperature was 25.0 °C, what would the final temperature of the gas be? Given: Equation: 4. A sample of gas occupies 2.00 l with 5.00 moles present. What would happen to the volume if the number of moles is increased to 10.0? Given: Equation:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
Name: Date: Period: 3/23 - 3/27 Assignment 1: Gas Law Practice Problems 1. A gas occupies 12.3 liter...
Questions



History, 16.12.2021 06:30


Law, 16.12.2021 06:30

Chemistry, 16.12.2021 06:30


Mathematics, 16.12.2021 06:30




English, 16.12.2021 06:30

Biology, 16.12.2021 06:30

World Languages, 16.12.2021 06:30


Physics, 16.12.2021 06:30

History, 16.12.2021 06:30

Social Studies, 16.12.2021 06:30


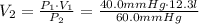 = 8.2 l
= 8.2 l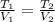
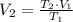 =
= 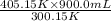 = 1214.84 ml
= 1214.84 ml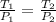
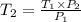
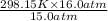 = 318.027 K
= 318.027 K

