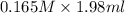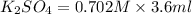Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
When aqueous solutions of K2SO4 and Pb(NO3)2 are combined, PbSO4 precipitates. Calculate the mass, i...
Questions


Biology, 21.02.2020 05:10


Health, 21.02.2020 05:10


Mathematics, 21.02.2020 05:10



Biology, 21.02.2020 05:10


Mathematics, 21.02.2020 05:10


Mathematics, 21.02.2020 05:10




Computers and Technology, 21.02.2020 05:11


English, 21.02.2020 05:11

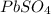 produced is 99.1 g.
produced is 99.1 g.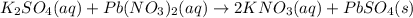
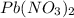 = Molarity × volume
= Molarity × volume