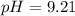Chemistry, 26.03.2020 17:49 seaotter9630
A chemist titrates 90.0 mL of a 0.5870 M acetic acid (HCH3CO2) solution with 0.4794M NaOH solution at 25 °C. Calculate the pH at equivalence. The p Kg of acetic acid is 4.76. Round your answer to 2 decimal places.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

You know the right answer?
A chemist titrates 90.0 mL of a 0.5870 M acetic acid (HCH3CO2) solution with 0.4794M NaOH solution a...
Questions

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Biology, 15.09.2020 01:01

Chemistry, 15.09.2020 01:01

Geography, 15.09.2020 01:01

Spanish, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

English, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

French, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

History, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

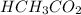 = 0.5870 M
= 0.5870 M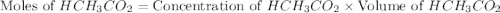
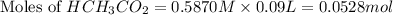
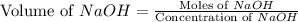
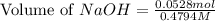
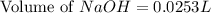
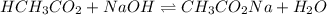
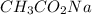 = 0.0528 mol
= 0.0528 mol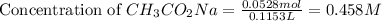
![pH=\frac{1}{2}[pK_w+pK_a+\log C]](/tpl/images/0565/3060/b44e5.png)
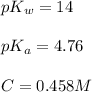
![pH=\frac{1}{2}[14+4.76+\log (0.458)]](/tpl/images/0565/3060/e66f0.png)