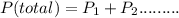Chemistry, 26.03.2020 16:46 manarsadi6
1. A sample of oxygen is collected over water at 22 ° C and 762 torr. What is the partial pressure of the dry oxygen? The vapor pressure of water at 22°C is 19.8 torr. A. 742 torr B. 782 torr C. 784 torr D. 750. Torr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
1. A sample of oxygen is collected over water at 22 ° C and 762 torr. What is the partial pressure o...
Questions












Mathematics, 16.07.2020 20:01