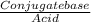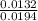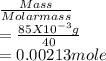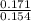Chemistry, 26.03.2020 05:19 kkjones1536
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH of this solution?Part B: What is the pH after addition of 150.0 mg of HBr?Part C: What is the pH after addition of 85.0 mg of NaOH?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH o...
Questions

Biology, 11.07.2019 05:00

English, 11.07.2019 05:00





Business, 11.07.2019 05:00




Business, 11.07.2019 05:00


Chemistry, 11.07.2019 05:00

Mathematics, 11.07.2019 05:00

Social Studies, 11.07.2019 05:00


Mathematics, 11.07.2019 05:00

Physics, 11.07.2019 05:00


History, 11.07.2019 05:00

![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0564/8323/225c1.png)
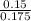
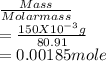
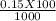 = 0.015
= 0.015 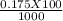 = 0.0175
= 0.0175