
Chemistry, 26.03.2020 04:28 jeffffffff
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution's temperature increases by 19.2°C. Calculate ∆H for the dissolution of the unknown solid. (The specific heat of the solution is 4.18 J/g・°C and the density of the solution is 1.20 g/mL).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1


Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution'...
Questions

Spanish, 27.10.2020 23:50

Mathematics, 27.10.2020 23:50

Biology, 27.10.2020 23:50


Chemistry, 27.10.2020 23:50

History, 27.10.2020 23:50





Mathematics, 27.10.2020 23:50

Health, 27.10.2020 23:50


Mathematics, 27.10.2020 23:50

Mathematics, 27.10.2020 23:50

French, 27.10.2020 23:50


Mathematics, 27.10.2020 23:50


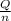 =
= 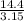 = 4.6 kj/mole
= 4.6 kj/mole

