
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a closed vessel at 400 k is charged with 1.00 atm of br2 (g), 1.00 atm of cl2 (g), and 2.00 atm of brcl (g). use q to determine which of the statements below is true.
a. the equilibrium partial pressure of br2 will be greater than 1.00 atm.
b. the reaction will go to completion since there are equal amounts of br2 and cl2.
c. the equilibrium partial pressure of brcl (g) will be greater than 2.00 atm.
d. the equilibrium partial pressures of br2, cl2, and brcl will be the same as the initial values.
e. at equilibrium, the total pressure in the vessel will be less than the initial total pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a close...
Questions

Mathematics, 08.05.2021 01:30



Mathematics, 08.05.2021 01:30

Mathematics, 08.05.2021 01:30


Mathematics, 08.05.2021 01:30






Chemistry, 08.05.2021 01:30

Health, 08.05.2021 01:30

Physics, 08.05.2021 01:30

Mathematics, 08.05.2021 01:30

Mathematics, 08.05.2021 01:30

History, 08.05.2021 01:30

French, 08.05.2021 01:30

Mathematics, 08.05.2021 01:30

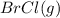 will be greater than
will be greater than 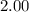 atm.
atm.![Q_{p} =\frac{[products]}{[reactants]}](/tpl/images/0564/6775/77b1b.png)
![Q_{p} = \frac{[2.00]^2}{{[1.00]}[1.00]}](/tpl/images/0564/6775/b1f67.png)
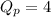
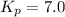
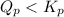 .
.

