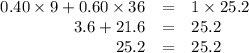Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
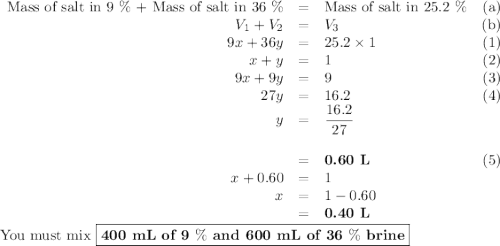
A biologist has two brine solutions, one containing 9% salt and another containing 36% salt. How man...
Questions


History, 10.03.2020 04:04


Biology, 10.03.2020 04:04

Mathematics, 10.03.2020 04:04

History, 10.03.2020 04:04







History, 10.03.2020 04:04

Spanish, 10.03.2020 04:04



Mathematics, 10.03.2020 04:04

Social Studies, 10.03.2020 04:04

Physics, 10.03.2020 04:04