Chemistry, 26.03.2020 02:59 lildeb8593
A cube of solid benzene (C6H6) at its melting point and weighing 10.0 g is placed in 10.0 g of water at 30 °C. Given that the heat of fusion of benzene is 9.92 kJ/mol, to what temperature will the water have cooled by the time all of the benzene has melted?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
A cube of solid benzene (C6H6) at its melting point and weighing 10.0 g is placed in 10.0 g of water...
Questions


Chemistry, 05.05.2020 02:13




History, 05.05.2020 02:13

Mathematics, 05.05.2020 02:13



History, 05.05.2020 02:13


Arts, 05.05.2020 02:13


Spanish, 05.05.2020 02:13

Chemistry, 05.05.2020 02:13

English, 05.05.2020 02:13

Mathematics, 05.05.2020 02:13

History, 05.05.2020 02:13


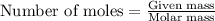
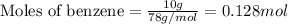
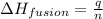
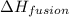 = heat of fusion of benzene = 9.92 kJ/mol = 9920 J/mol (Conversion factor: 1 kJ = 1000 J)
= heat of fusion of benzene = 9.92 kJ/mol = 9920 J/mol (Conversion factor: 1 kJ = 1000 J)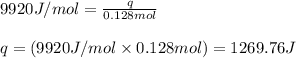
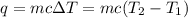
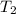 = final temperature = ?
= final temperature = ?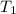 = initial temperature = 30°C
= initial temperature = 30°C