

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
The rate constant for the reaction is 0.660 M − 1 ⋅ s − 1 0.660 M−1⋅s−1 at 200 ∘ C. 200 ∘C. A ⟶ prod...
Questions

Physics, 26.04.2021 23:00

Business, 26.04.2021 23:00



Advanced Placement (AP), 26.04.2021 23:00

Chemistry, 26.04.2021 23:00

Chemistry, 26.04.2021 23:00





Spanish, 26.04.2021 23:00


Mathematics, 26.04.2021 23:00






![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0564/2226/5ea71.png)
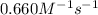
![[A]_o](/tpl/images/0564/2226/9caf5.png) = Initial concentration = 0.00440 M
= Initial concentration = 0.00440 M![0.660=\frac{1}{865}\left (\frac{1}{[A]}-\frac{1}{(0.00440)}\right)](/tpl/images/0564/2226/9d3b6.png)
![[A]=0.00125M](/tpl/images/0564/2226/63ab7.png)



