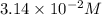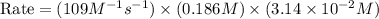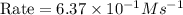Chemistry, 25.03.2020 23:55 markusovaevelyn532
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first order in O3. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate =
In an experiment to determine the rate law, the rate constant was determined to be 109 M-1s-1. Using this value for the rate constant, the rate of the reaction when [NO] = 0.186 M and [O3] = 3.14×10-2 M would be [blank] Ms-1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2
You know the right answer?
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first...
Questions

Mathematics, 16.09.2019 14:00

Social Studies, 16.09.2019 14:00


Social Studies, 16.09.2019 14:10

English, 16.09.2019 14:10


History, 16.09.2019 14:10

History, 16.09.2019 14:10


History, 16.09.2019 14:10

Mathematics, 16.09.2019 14:10

Biology, 16.09.2019 14:10

Mathematics, 16.09.2019 14:10



Mathematics, 16.09.2019 14:10

Chemistry, 16.09.2019 14:10

Mathematics, 16.09.2019 14:10


Biology, 16.09.2019 14:10

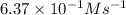
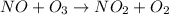
![\text{Rate}=k[NO]^a[O_3]^b](/tpl/images/0564/1543/2796d.png)
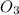 = 1
= 1![\text{Rate}=k[NO]^1[O_3]^1](/tpl/images/0564/1543/85a0b.png)
![\text{Rate}=k[NO][O_3]](/tpl/images/0564/1543/1a568.png)
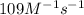
![[O_3]](/tpl/images/0564/1543/8b13e.png) = concentration of
= concentration of