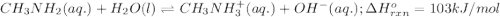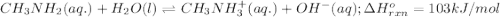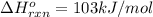Chemistry, 25.03.2020 22:37 kamjay2006
Use Le Châtelier's principle to predict how the equilibrium for the weak base methylamine responds to the indicated changes. CH 3 NH 2 ( aq ) + H 2 O ( l ) − ⇀ ↽ − CH 3 NH + 3 ( aq ) + OH − ( aq ) Δ H ∘ rxn = 103 kJ / mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

You know the right answer?
Use Le Châtelier's principle to predict how the equilibrium for the weak base methylamine responds t...
Questions



Mathematics, 23.03.2021 16:40

Advanced Placement (AP), 23.03.2021 16:40

History, 23.03.2021 16:40

Mathematics, 23.03.2021 16:40

Mathematics, 23.03.2021 16:40

Mathematics, 23.03.2021 16:40






Mathematics, 23.03.2021 16:40






English, 23.03.2021 16:40