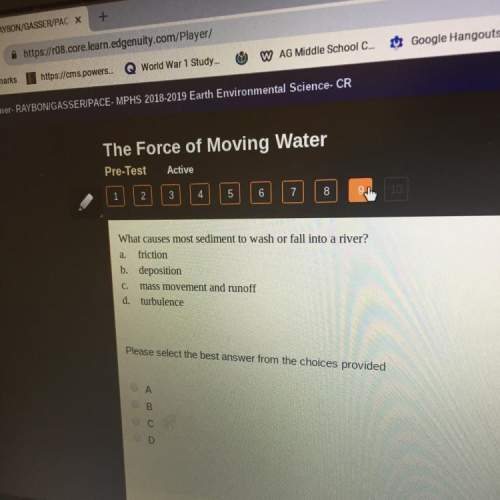
Read the following chemical equation.
Fe(s) + O2 (g) → Fe3+ O2−
HELP PLS!!!
...

Chemistry, 25.03.2020 21:49 krystlemiller4307
Read the following chemical equation.
Fe(s) + O2 (g) → Fe3+ O2−
HELP PLS!!!
What most likely happens during this reaction?
Iron loses three electrons.
Iron gains three electrons.
Oxygen loses one electron.
Oxygen gains one electron.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Questions

Mathematics, 26.04.2020 05:07

Mathematics, 26.04.2020 05:07


Mathematics, 26.04.2020 05:07

English, 26.04.2020 05:07


Biology, 26.04.2020 05:08



Social Studies, 26.04.2020 05:08


History, 26.04.2020 05:08





Mathematics, 26.04.2020 05:08

Mathematics, 26.04.2020 05:08

History, 26.04.2020 05:08

Mathematics, 26.04.2020 05:09