
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The heat of neutralization for the formation of water -55.9 kJ/mole. The heat of solvation (it might be called the heat of solution) for sodium hydroxide is 41.0 kJ/mole What quantity of heat is released?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The hea...
Questions


History, 11.12.2019 12:31

Mathematics, 11.12.2019 12:31






Advanced Placement (AP), 11.12.2019 12:31

Physics, 11.12.2019 12:31

Biology, 11.12.2019 12:31

Mathematics, 11.12.2019 12:31


Social Studies, 11.12.2019 12:31






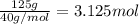

 of water
of water



