

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

You know the right answer?
In 1901, Thomas Edison invented the nickel-iron battery. The follwing reaction takes place in the ba...
Questions




Mathematics, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00



SAT, 15.09.2021 14:00

Chemistry, 15.09.2021 14:00




Mathematics, 15.09.2021 14:00

Social Studies, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00


Physics, 15.09.2021 14:00

English, 15.09.2021 14:00

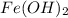 produced are, 3.60 moles.
produced are, 3.60 moles.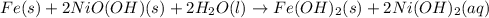
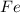 react with 2 mole of
react with 2 mole of 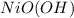
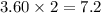 moles of
moles of 

