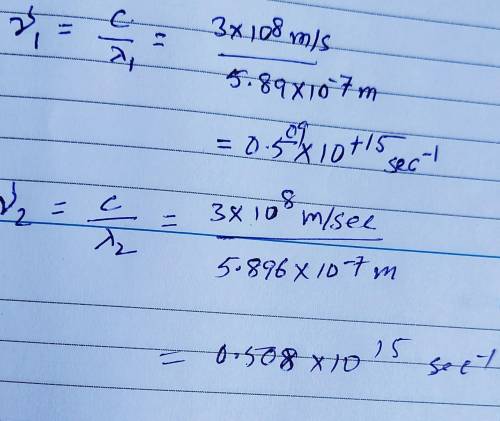Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by these lamps is actually due to two closely spaced emission lines in the visible region of the electromagnetic spectrum. One of these lines has a wavelength of 5.890 X 10⁻⁷ m, and the other line has a wavelength of 5.896 X 10⁻⁷ m. A) What are the wavelengths of these radiations in centimeters? B) Calculate the frequencies of these radiations. Show work please

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by the...
Questions



Mathematics, 19.09.2019 21:10


Mathematics, 19.09.2019 21:10

Biology, 19.09.2019 21:10




Mathematics, 19.09.2019 21:10

English, 19.09.2019 21:10




Physics, 19.09.2019 21:10


Geography, 19.09.2019 21:10


Social Studies, 19.09.2019 21:10