Chemistry, 25.03.2020 14:59 marahkotelman
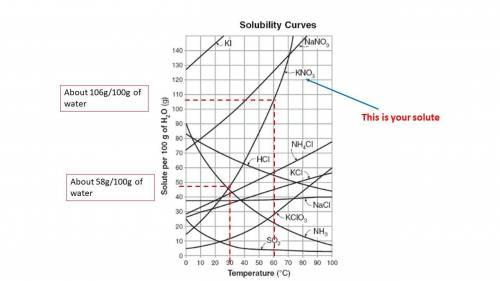
A saturated solution of KNO3 at 60C was cooled to 30C. How many grams of KI will come out of the solution as solid and settle at the bottom of the solution undissolved? Hints: Take the solubility of KNO3 at both temperatures and find the difference.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A saturated solution of KNO3 at 60C was cooled to 30C. How many grams of KI will come out of the sol...
Questions



Mathematics, 20.09.2019 04:10


Mathematics, 20.09.2019 04:10

Mathematics, 20.09.2019 04:10



Biology, 20.09.2019 04:10

Mathematics, 20.09.2019 04:10


Social Studies, 20.09.2019 04:10