
Chemistry, 25.03.2020 06:21 miacespedes
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentration of N2O of 0.50 M, calculate the concentration of N2O remaining after 2.0 min if k = 3.4 × 10−3 s −1 . 1. 0.17 M 2. 0.50 M 3. 0.55 M 4. 0.33 M 5. 0.66 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentra...
Questions








Computers and Technology, 17.02.2020 17:14



Mathematics, 17.02.2020 17:15


Computers and Technology, 17.02.2020 17:15



Computers and Technology, 17.02.2020 17:16



Geography, 17.02.2020 17:17

English, 17.02.2020 17:17

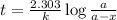
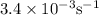
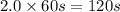
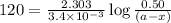
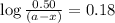
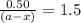
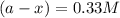
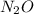 remaining after 2.0 min is 0.33 M
remaining after 2.0 min is 0.33 M

