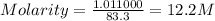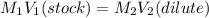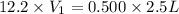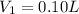Chemistry, 25.03.2020 05:31 wallsdeandre25521
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass and a density of 1.20 g / mL. How much of the concentrated stock solution in milliliters should you use to make 2.5 L of 0.500 M HCl

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass a...
Questions




Mathematics, 18.05.2021 01:00

Mathematics, 18.05.2021 01:00

Health, 18.05.2021 01:00





Chemistry, 18.05.2021 01:00

Social Studies, 18.05.2021 01:00


Mathematics, 18.05.2021 01:00


Mathematics, 18.05.2021 01:00



Mathematics, 18.05.2021 01:00

Arts, 18.05.2021 01:00

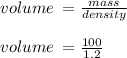
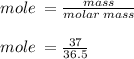
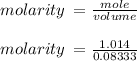
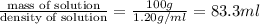
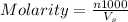
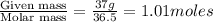
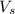 = volume of solution in ml = 83.3 ml
= volume of solution in ml = 83.3 ml