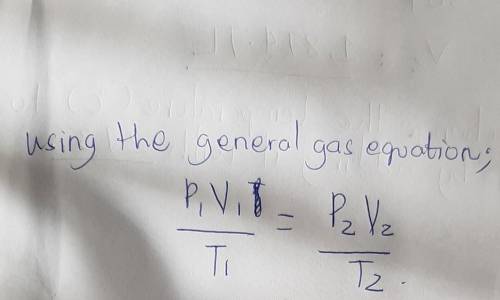Chemistry, 25.03.2020 05:33 devenybates
A sample of gas with an initial volume of 27.9 L at a pressure of 721 mmHg and a temperature of 309 K is compressed to a volume of 15.5 L and warmed to a temperature of 377 K .
What is the final pressure of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1


You know the right answer?
A sample of gas with an initial volume of 27.9 L at a pressure of 721 mmHg and a temperature of 309...
Questions

Mathematics, 05.12.2020 03:40

English, 05.12.2020 03:40





English, 05.12.2020 03:40

Mathematics, 05.12.2020 03:40

History, 05.12.2020 03:40







Law, 05.12.2020 03:40


Mathematics, 05.12.2020 03:40


Mathematics, 05.12.2020 03:40