

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Which statement is true of water? Water molecules attract hydrophobic substances. Water evaporates a...
Questions


Health, 20.09.2019 04:00

Social Studies, 20.09.2019 04:00

History, 20.09.2019 04:00


Mathematics, 20.09.2019 04:00



Health, 20.09.2019 04:00

Mathematics, 20.09.2019 04:00

Mathematics, 20.09.2019 04:00

English, 20.09.2019 04:00

Mathematics, 20.09.2019 04:00


Social Studies, 20.09.2019 04:00

Biology, 20.09.2019 04:00


Mathematics, 20.09.2019 04:00


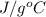 . Therefore, in order to evaporate it needs to absorb high heat and not small amount of heat.
. Therefore, in order to evaporate it needs to absorb high heat and not small amount of heat.

