Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
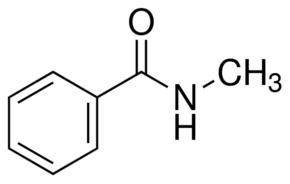
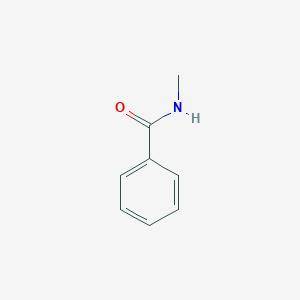
N-methylbenzamide, an isomer of acetanilide, when allowed to react with HNO3/h2so4 gives a different...
Questions

Computers and Technology, 07.10.2020 06:01



English, 07.10.2020 06:01

Mathematics, 07.10.2020 06:01


Mathematics, 07.10.2020 06:01





Physics, 07.10.2020 06:01

English, 07.10.2020 06:01






Social Studies, 07.10.2020 06:01