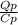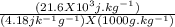Chemistry, 25.03.2020 05:45 lizredrose5
Assume the weight of an average adult is 70. kg, and that 420. kJ of heat are evolved per mole of oxygen consumed as a result of the oxidation of foodstuffs, knowing that the number of moles of O2 inhaled per minutes are 0.02 mol/min. Suppose an adult body were encased in a thermally insulating barrier. If as a result of this barrier all the heat evolved by metabolism of foodstuffs were retained by the body, what would the temperature difference of the body reach after 3 hours

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 02:30
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

You know the right answer?
Assume the weight of an average adult is 70. kg, and that 420. kJ of heat are evolved per mole of ox...
Questions

Mathematics, 27.10.2020 05:40



Mathematics, 27.10.2020 05:40

Mathematics, 27.10.2020 05:40

Physics, 27.10.2020 05:40

Mathematics, 27.10.2020 05:40

Mathematics, 27.10.2020 05:40


Mathematics, 27.10.2020 05:40

History, 27.10.2020 05:40

Mathematics, 27.10.2020 05:40

Mathematics, 27.10.2020 05:40

English, 27.10.2020 05:40



Mathematics, 27.10.2020 05:50



Mathematics, 27.10.2020 05:50

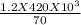 = 7.2 kj.h⁻¹.Kg⁻¹
= 7.2 kj.h⁻¹.Kg⁻¹