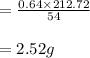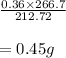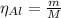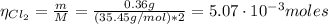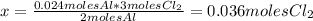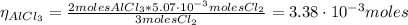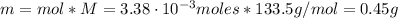Chemistry, 25.03.2020 05:47 ruchierosanp1n3qw
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.64 g Al is mixed with 0.36 g Cl2. (a) What is the limiting reactant? Al Cl2 (b) What is the maximum amount of AlCl3, in grams, that can be produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlC...
Questions

Chemistry, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50

Computers and Technology, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50


English, 27.10.2020 15:50


English, 27.10.2020 15:50


Mathematics, 27.10.2020 15:50


Mathematics, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50

History, 27.10.2020 15:50


Mathematics, 27.10.2020 15:50

Business, 27.10.2020 15:50

Mathematics, 27.10.2020 15:50