
Chemistry, 25.03.2020 05:51 kokokakahi
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30 M , and [C] = 0.500 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.410 M and [C] = 0.690 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1
You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30...
Questions

Mathematics, 11.07.2019 07:00

Mathematics, 11.07.2019 07:00



Computers and Technology, 11.07.2019 07:00

Biology, 11.07.2019 07:00

History, 11.07.2019 07:00


Mathematics, 11.07.2019 07:00

Mathematics, 11.07.2019 07:00



Business, 11.07.2019 07:00


Social Studies, 11.07.2019 07:00

Mathematics, 11.07.2019 07:00




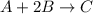
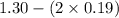
![K_{eq} = \frac{[C]}{[A][B]^{2}}](/tpl/images/0562/6350/666b5.png)
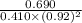
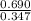
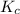 is 1.988.
is 1.988.

