
Chemistry, 25.03.2020 05:05 chonawilson4
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nitrogen dioxide is also produced in the reaction.) What is the enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature (R = 0.0821 L atm/mol K)? [ΔH°f(NO) = 90.4 kJ/mol; ΔH°f(NO2) = 33.85 kJ/mol; ΔH°f(O3) = 142.2 kJ/mol]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3


Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1
You know the right answer?
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nit...
Questions

World Languages, 25.08.2019 20:30



Mathematics, 25.08.2019 20:30



History, 25.08.2019 20:30

Mathematics, 25.08.2019 20:30


Social Studies, 25.08.2019 20:30


Advanced Placement (AP), 25.08.2019 20:30

Mathematics, 25.08.2019 20:30

Geography, 25.08.2019 20:30


Biology, 25.08.2019 20:30


Mathematics, 25.08.2019 20:30


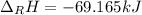
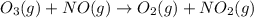
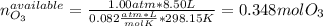
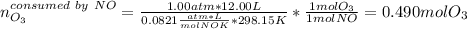
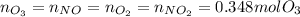
![\Delta _RH=n[\Delta _fH_{products}-\Delta _fH_{reagents}]\\\Delta _RH=0.348mol*[33.85 kJ/mol+0kJ/mol-142.2 kJ/mol-90.4 kJ/mol]\\\Delta _RH=-69.165kJ](/tpl/images/0562/4480/3a1de.png)




