

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
Consider the following reaction: 2SO2(g)+O2(g)⇌2SO3(g) Kp=0.355 at 950 K A 2.75−L reaction vessel at...
Questions

History, 25.08.2020 09:01

English, 25.08.2020 09:01

Mathematics, 25.08.2020 09:01




Mathematics, 25.08.2020 09:01

Mathematics, 25.08.2020 09:01

Mathematics, 25.08.2020 09:01

Computers and Technology, 25.08.2020 09:01

History, 25.08.2020 09:01


Mathematics, 25.08.2020 09:01



Mathematics, 25.08.2020 09:01

Physics, 25.08.2020 09:01



History, 25.08.2020 09:01

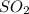 and
and 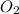
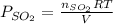
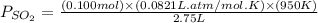
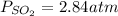
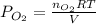
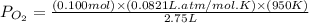
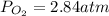
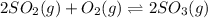
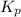 for above reaction follows:
for above reaction follows: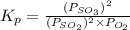
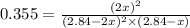
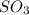 = (2x) = 2(0.664) = 1.33 atm
= (2x) = 2(0.664) = 1.33 atm

