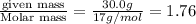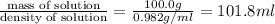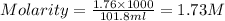Chemistry, 25.03.2020 03:47 haileywatkins
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at 25 °C. The density of water at that temperature is 0.982 g mL−1. M(NH3) = 17.034 g mol−1 A. 17.3 mol L−1 B. 24.7 mol L−1 C. 5.78 × 10−2 mol L−1 D. 0.578 mol L−1 E. 1.73 mol L−1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
3. Calculate the molarity of an NH3 aqueous solution made up of 30.0 g of NH3 and 70.0 g of water at...
Questions



Mathematics, 11.04.2021 03:20

Advanced Placement (AP), 11.04.2021 03:20


Business, 11.04.2021 03:30

Computers and Technology, 11.04.2021 03:30

Mathematics, 11.04.2021 03:30






Mathematics, 11.04.2021 03:30

Biology, 11.04.2021 03:30


Computers and Technology, 11.04.2021 03:30



English, 11.04.2021 03:30

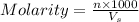
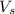 = volume of solution in ml
= volume of solution in ml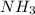 (solute) =
(solute) =