Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° = -90 kJ
II) Ni(s) + 4 CO(g) = Ni(CO)4(8),
AH° = -161 kJ
will shift toward products or reactants with a
temperature increase.
1. I shifts toward reactants and II shifts
toward products.
2. Both I and II shift toward products.
3. Unable to determine
4. Both I and II shift toward reactants.
5. I shifts toward products and II shifts to-
ward reactants.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
Questions

Mathematics, 31.10.2019 20:31

Biology, 31.10.2019 20:31





Mathematics, 31.10.2019 20:31






English, 31.10.2019 20:31


Mathematics, 31.10.2019 20:31


Mathematics, 31.10.2019 20:31


Mathematics, 31.10.2019 20:31

History, 31.10.2019 20:31

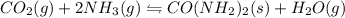 ,ΔH° = -90 kJ
,ΔH° = -90 kJ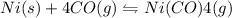 ,ΔH° = -161 kJ
,ΔH° = -161 kJ