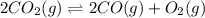Chemistry, 25.03.2020 02:24 khenalilovespandas
When each of the following equilibria is disturbed by increasing the pressure as a result of decreasing the volume, does the number of moles of reaction products increase, decrease, or remain the same? Part A 2CO2(g)⇌2CO(g)+O2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

You know the right answer?
When each of the following equilibria is disturbed by increasing the pressure as a result of decreas...
Questions




Physics, 24.07.2019 02:30

Physics, 24.07.2019 02:30

Physics, 24.07.2019 02:30


Physics, 24.07.2019 02:30



Computers and Technology, 24.07.2019 02:30

Physics, 24.07.2019 02:30


Chemistry, 24.07.2019 02:30



Mathematics, 24.07.2019 02:30



Computers and Technology, 24.07.2019 02:30