
A major component of gasoline is octane C8H18. When liquid octane is burned in air it reacts with oxygen O2 gas to produce carbon dioxide gas and water vapor. Calculate the moles of carbon dioxide produced by the reaction of 0.085mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
A major component of gasoline is octane C8H18. When liquid octane is burned in air it reacts with ox...
Questions

Mathematics, 29.01.2022 14:00


Biology, 29.01.2022 14:00


Mathematics, 29.01.2022 14:00

History, 29.01.2022 14:00


History, 29.01.2022 14:00

Mathematics, 29.01.2022 14:00


Arts, 29.01.2022 14:00


English, 29.01.2022 14:00

Mathematics, 29.01.2022 14:00

Chemistry, 29.01.2022 14:00

Biology, 29.01.2022 14:00




Computers and Technology, 29.01.2022 14:00

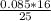 = 0.1 mole of CO₂.
= 0.1 mole of CO₂. 

