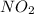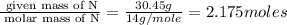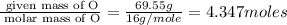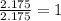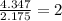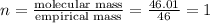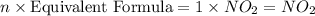Chemistry, 25.03.2020 02:00 AlaskaAirlines
A compound is found to contain 30.45 % nitrogen and 69.55 % oxygen by mass. To answer the question, enter the elements in the order presented above. QUESTION 1: The empirical formula for this compound is . QUESTION 2: The molar mass for this compound is 46.01 g/mol. The molecular formula for this compound is .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
A compound is found to contain 30.45 % nitrogen and 69.55 % oxygen by mass. To answer the question,...
Questions

Biology, 20.01.2022 03:40



Chemistry, 20.01.2022 03:40




Mathematics, 20.01.2022 03:40



English, 20.01.2022 03:40


Social Studies, 20.01.2022 03:40



Mathematics, 20.01.2022 03:40

Computers and Technology, 20.01.2022 03:40

Mathematics, 20.01.2022 03:40