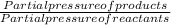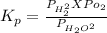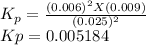The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperature until the partial pressures of H 2 O , H 2 , and O 2 reach 0.025 atm, 0.0060 atm, and 0.0090 atm, respectively at equilibrium. What is the value of the equilibrium constant at this temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1


Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperat...
Questions


Mathematics, 02.07.2020 16:01

English, 02.07.2020 16:01

History, 02.07.2020 16:01


Physics, 02.07.2020 16:01

Mathematics, 02.07.2020 16:01





Mathematics, 02.07.2020 16:01

Mathematics, 02.07.2020 16:01


Mathematics, 02.07.2020 16:01



Mathematics, 02.07.2020 16:01

English, 02.07.2020 16:01

Mathematics, 02.07.2020 16:01